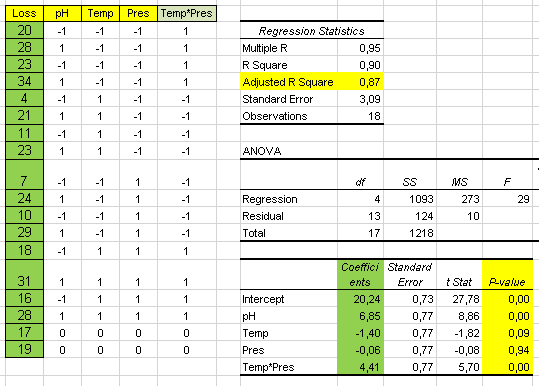
Water Engine Fallacy
Some Historical Claims
Multiple "inventors" worldwide have claimed the invenction of engines which run on water alone.
Even nowadays some companys claim they can do it.
These are some of the claims:
- With the water engine you don't need batteries.
- You don't need to carry hydrogen cells.
- All you need is water.
- Vested interests of large fosil fuel multinationals are making sure water engine doesn't succeed.
An engine that runs just on water
Obtain Hydrogen from Water
Electrolysis Energy Requirements
To obtain 1 kilogram of hydrogen from water through electrolysis, it typically requires around 50 to 55 kilowatt-hours (kWh) of electric energy.
Here's a breakdown of the calculation:
Energy Required for Electrolysis: The electrolysis of water to produce hydrogen involves splitting water (H₂O) into hydrogen (H₂) and oxygen (O₂). The theoretical minimum energy required is about 39.4 kWh per kilogram of hydrogen under ideal conditions.
Efficiency of the Electrolyzer: In practice, electrolyzers are not 100% efficient. Modern electrolyzers usually have efficiencies between 65% and 80%. So, to account for inefficiencies, you multiply the theoretical energy requirement by the inverse of the efficiency.
This range accounts for different types of electrolyzers and their varying efficiencies.
Run an Internal Combustion Engine with Hydrogen
The amount of energy an internal combustion engine (ICE) can deliver from 1 kilogram of hydrogen depends on several factors, including the efficiency of the engine and the energy content of hydrogen.
Key Points:
- Energy
Content of Hydrogen:
- Hydrogen has a high energy content by weight, with a lower heating value (LHV) of approximately 33.33 kWh per kilogram.
- Efficiency
of an Internal Combustion Engine (ICE):
- Internal
combustion engines are typically less efficient than fuel cells. The
efficiency of a hydrogen-powered ICE can vary, but it is usually between 20%
and 30%.
Calculation:
To find out how many kilowatt-hours an ICE can deliver from
1 kg of hydrogen:
- Energy
delivered by the engine = Energy content of hydrogen × Efficiency of
the ICE
- Using
a typical efficiency range:
Energy delivered = 33.33 kWh/kg×(0.20 to 0.30) = 6.67 to 10kWh
Efficiency of the Water Engine
Current Efficiency of an Electric Car
Fuel Cell Alternative
Car Power Alternatives
Conclusions:
- The so called "Water Engine" doesn't run on water but on electricity.
- The "Water Engine" is, therefore, a very poor performance electric engine.
- The efficiency of this "Water Engine" is 25% of normal electric engines.
- Therefore this "Water Engine" requires to carry batteries 4 times larger than an electric car.
- The folly of water-fuelled vehicles











Comments
Post a Comment